Walked closely with the global advancement of pelvic floor rehabilitation technology.
With expansion of business operation, progression in technology, the company had been recognized by the global market.
By steady growth and continuous technology developments, the company had gained strong positions in the global market.
Facing fierce market competitions, the company has been enhancing investments into technology development, introducing new products continuously, and leading the market trends.

Began the development of the world's first all-digital 6-mode female pelvic floor training device "Stim Gym Lady", which provides solutions for the treatment of different types of urinary incontinence and pelvic floor training functions. The product development was completed in February 2002.

Walked closely with the global advancement of pelvic floor rehabilitation technology, EasyMed was established.

EasyMed was awarded the certificate of ISO 13485 by TUV Rheinland of Germany, the quality management system required for medical product providers under international stands. The company has been keeping up with the requirements of such standards since then.
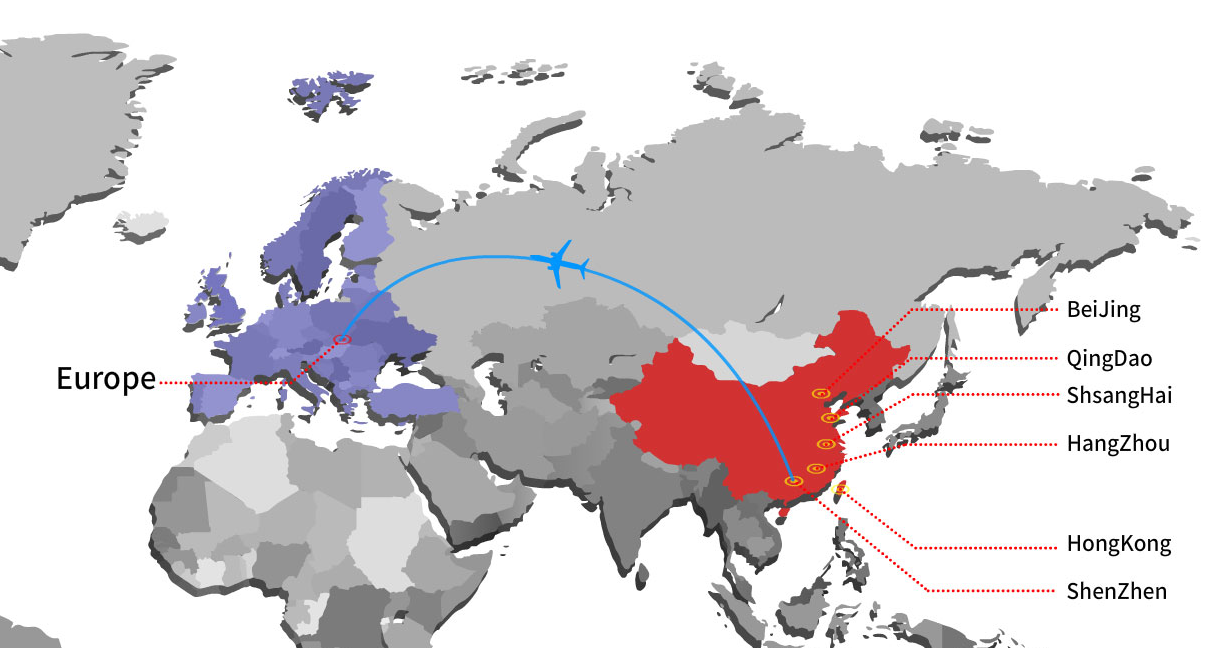
The first shipment of products manufactured by EasyMed was departed to Europe.

Successfully developed the Chinese version of pelvic floor muscle rehabilitation devices both for in home use (Model: NSJ-01) and for hospital settings (Model: NSJ-02). At the same time, completed development of the key accessories of the device, including the EMG sensor with biofeedback, the first of its kind in China.
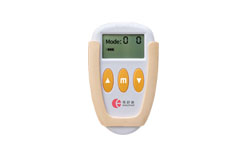
Dysmenorrhea pain relief unit WM-01 was registered with CFDA of China.

EasyMed received its first product approval by the US FDA, allowing the product to enter the commerce of the US market.

The company was awarded the certification of CMDCAS by TUV Rheinland of North America.

Deep vein embolization therapy(DVT) device was successfully developed.
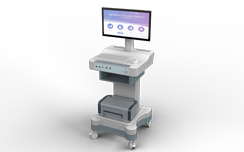
NSJ-01 and NSJ-02 (urinary incontinence treatment device) was registered successfully with Guangdong Food and Drug Administration: 2004 No. 2630219.
In 2017, the China National Food and Drug Administration approved the registration and the product was renamed as "Pelvic Floor Muscle Rehabilitation Therapy Device" based on product characteristics, the product thus became the first registered certificate in China under this name.

Developed the European version of intelligent pelvic floor stimulator, "Intellistim UG", and obtained CE certification for the product.

EasyMed obtained the US FDA approval for the second product, and began to supply to the market in the United States of America.

The company developed its first product to relief Labor pain for women during delivery.

Developed the European version of the digital pelvic floor stimulator "Logistim UG", and obtained CE certification.
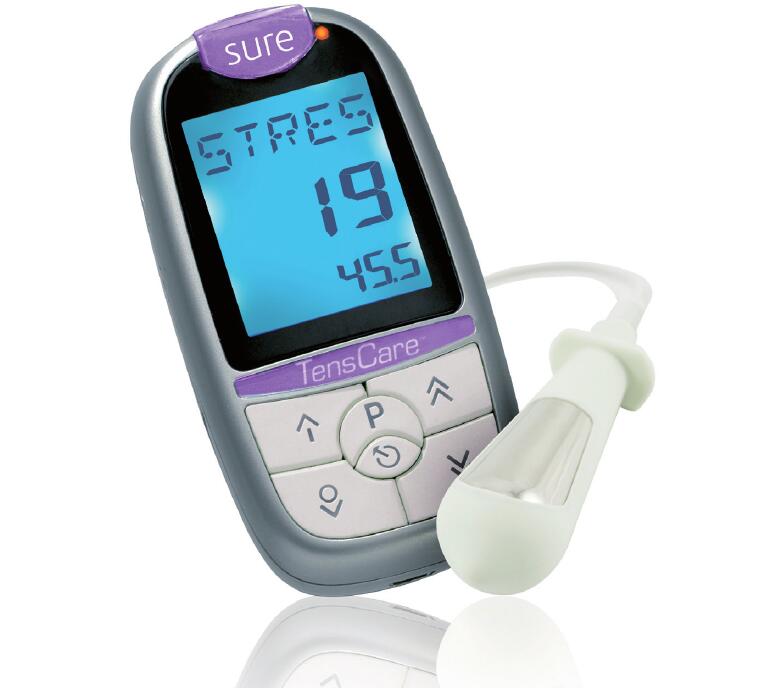
Successfully developed "Sure", the European version of the "Perfect" pelvic floor trainer, and obtained CE certification. The product was also approved by FDA 510(k) certification in April, 2011.
With a huge advancement from many products in the market which had serious discomfort during treatments, "Sure" produces a unique output characteristics that adapts comfortably to female body for the first time. This unique output characteristics is called "Jeffery's Output" and it has been widely praised by many users of the devices. Since then, Jeffery's output has been applied in a lot of products in today's market.

Supporting by the global growth of business, EasyMed moved to a new facility equipped with modern manufacturing technologies, which allows the company to supply our customers with better quality products continuously.

Created a Chinese brand of EasyMed for more effective marketing and services to our customers in China. The company strives to introducing advanced electrotherapies from Europe to all Chinese in need.

Completed the development of "KEAT Pro", a pelvic floor treatment device for the Europe, and was certified with CE mark.

Initiated the development of a peripheral nerve stimulator for monitoring anesthetized muscle relaxation.

Completed the development of the Chinese version of pelvic floor training device NSJ-01A.

Began the development of NEO, a four-in-one device with three-dimensional dynamic interference function.

Completed the development of the European version of male pelvic floor trainer, "KEAT MEN".

Completed the development of the European version of mini pelvic floor trainer, "Elise", which was designed with a compact size of 63 x 100 x 17.5mm for the first time, and obtained CE certification.

NEO, a four-in-one device with three-dimensional dynamic interference function, was approved by the US FDA for market entrance.
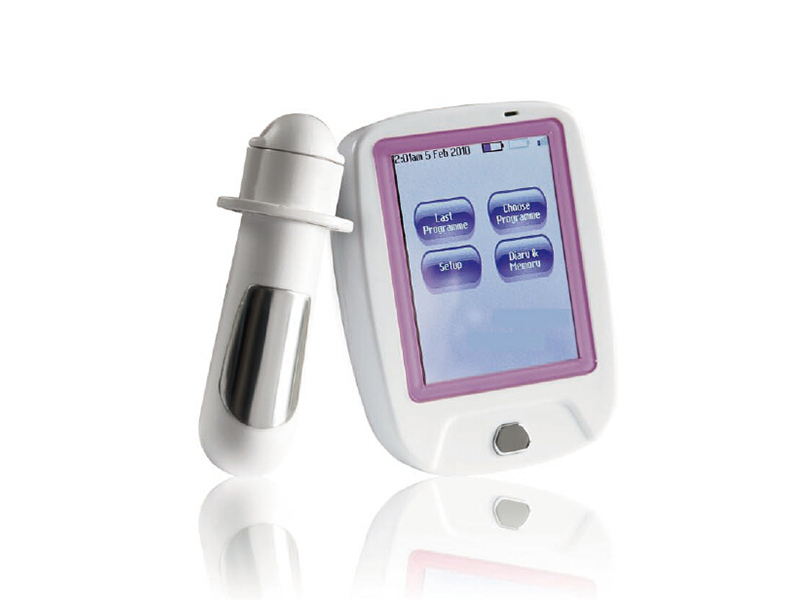
Completed the development of Viva, a wireless remote controlled pelvic floor therapy device, and obtained CE certification

EasyMed's peripheral nerve stimulator for monitoring anesthetized muscle relaxation was approved by the FDA for the entrance of commerce in the USA.

Started the research and development of smart devices remotely controlled by mobile phone APP through Bluetooth, and successfully completed in August 2014.

Completed the development of European version of the professional pelvic floor rehabilitation device "Sure Pro", and obtained CE certification.

Completed the development of the American version of the pelvic floor trainer "Softcycle" and entered the American market for the first time. Later, a Chinese version of the model was developed based on the same technology.

Completed the core product development of the European version of smart pelvic floor stimulator "evoStim UG".

The smart TENS/EMS device "iTENS", which is remotely controlled by the mobile phone APP via Bluetooth, was approved by the FDA for listing.

On April 17-21, 2016, the 75th China International Medical Equipment Fair (CMEF) was held at the Shanghai National Convention and Exhibition Center. EasyMed made its first appearance at the show, bringing a series of major products and new products to the exhibition.

The European version of the integrated pelvic floor device "Gyneffik " had been developed and CE certified

EasyMed participated in the 78th China International Medical Equipment (Autumn) Expo in 2017 and the 25th China International Medical Equipment Design and Manufacturing Technology (Autumn) Expo.

The 79th China International Medical Equipment (Spring) Expo and the 26th China International Medical Equipment Design and Manufacturing Technology (Spring) Exhibition kicked off at the National Convention and Exhibition Center (Shanghai), where EasyMed made its shining debut.

Completed the development of the professional Chinese version of pelvic floor muscle rehabilitation therapy device NSJ-03, and obtained the registration certificate from the CFDA.

EasyMed brought a series of major products and new products to the CIMEE exhibition. As a senior brand in China's medical device industry, we followed the theme of the conference and serviced guests from around the world at booth No. F23/F25/G40/G42 in Hall 8.

Continuous certification for CE mark quality system was awarded.

EasyMed was awarded the certification of MDSAP, which is a very strict quality management system for medical product suppliers, and it is recognized by the five countries' authorities, including USA, Canada, Australia, Japan, and Brazil.